
The potential for reinfection after being sick with SARS-CoV-2019 has me a little unsettled. I hope it's just a idiosyncratic case.
I had a bit of a freak-out back around 2005 when I learned about influenza pandemics and H5N1. I did all the things from buying basic supplies (and rotating food for years) to working with a friend online (over AIM w/trillian) to come up with an over-the-counter route to synthesize oseltamivir (tamiflu).
For example the starting material, furan, can be sourced from corn cobs (furfural from corncobs, 2-furancarboxylic acid from furfural, furan from 2-furancarboxylic acid). Or, far more feasibly, just extracted from it's use as a solvent from a commercial product. The current pandemic reminds me of that fun but wasted effort. It's presented below so someone might get a kick out of it.
OTC Oseltamiver Synthesis
By Chris The Great, 2006
This document has not yet been tested to ensure that it works as presented.
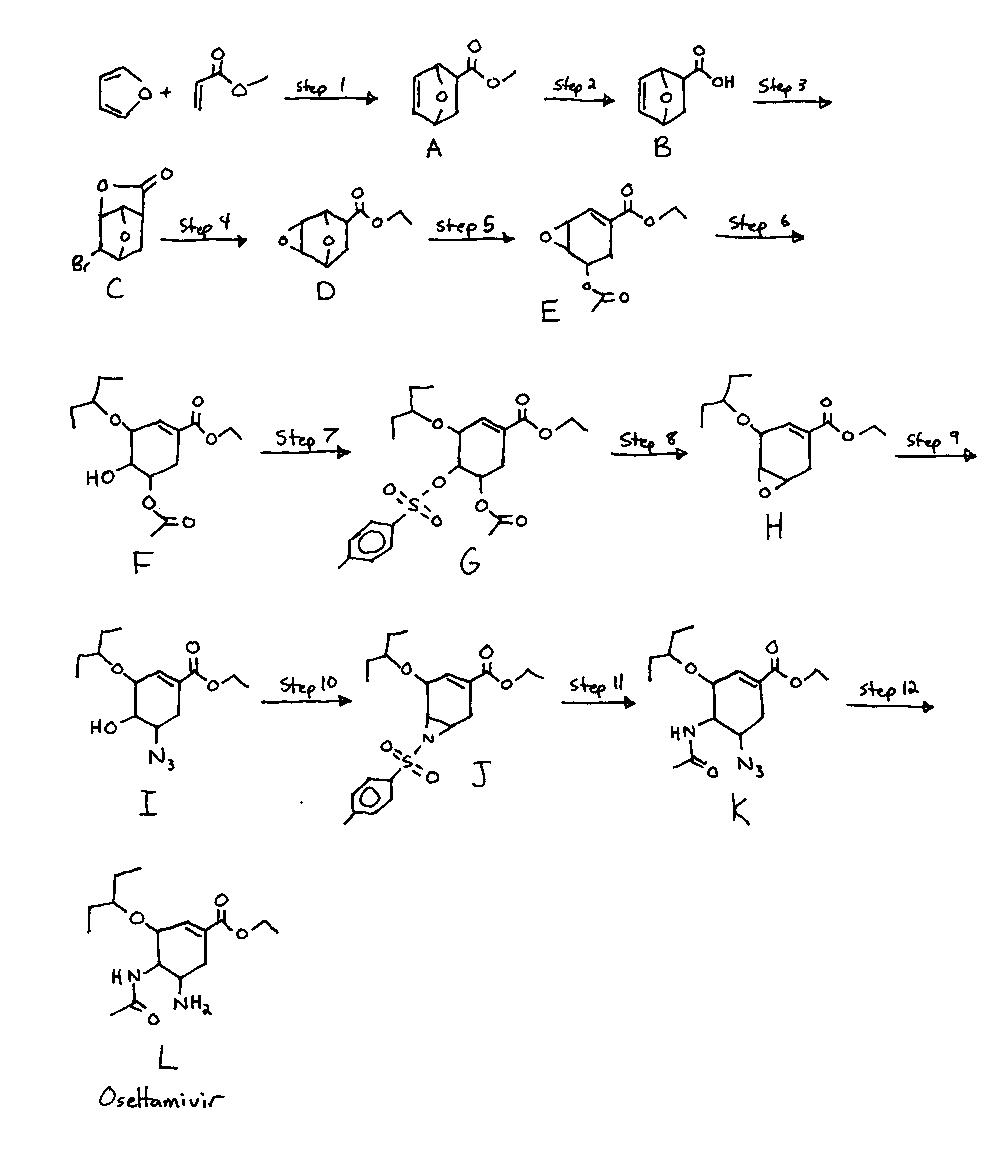 Step One: Synthesis of A
Methyl acrylate (2.78mol) is added to furan (3.95mol) and cooled to -10*C. AlCl3 in DCM (1.15mol in 1-5M conc.) is added slowly with stirring, keeping the temperature below -10*C. The mixture is then cooled with a cold water bath and stirred for 2 hours. The mixture is added to 1000ml of saturated sodium bicarbonate solution, and the precipitate is filtered out. The aqeous layer is extracted twice with 250ml DCM, and the organic layers are combined and dried over anhydrous sodium sulfate. The solvent is distilled away and the product purified by silica gel column chromatography to give the desired product in 38.6% yield (1.07mol).
Step Two: Synthesis of B
A (1.07mol) is added to NaOH (1.65mol, 3 to 15M concentration) in water with stirring, maintaining a temperature of 5*C. The mixture is allowed to warm up over a period of 30 minutes with stirring, and stirred at room temperature for 2 hours. HCl (310ml of 31% sol.) is added to adjust the pH to below 1, and then the mixture is extracted 4 times with 200ml of DCM. The combined organic layers are dried with Na2SO4, and solvent evaporated and the product recrystallized from ethyl acetate to give the desired product in 87.5% yield (0.936mol).
Step Three: Synthesis of C
B (0.936mol) is dissolved into 850ml water, and NaHCO3 (0.936mol) is added slowly, with stirring to control the foaming. After foaming ceases, bromine (0.936mol) is added dropwise with stirring, and then the mixture is stirred at room temperature for 2 hours. The mixture is extracted with 400ml ethyl acetate, washed with 100ml thiosulfate solution (to remove unreacted bromine) and dried with Na2SO4. The solvent is distilled away and the product purfied by recrystallizing from ethyl acetate, giving the desired product in 89.6% yeild (0.84mol)
Step Four: Synthesis of D
Potassium hydroxide (2.5mol) is dissolved into dimethylacetamide (1000ml) and methanol (150ml), and C (0.84mol) is added slowly as the mixture is heated to a gentle reflux. It is stirred under reflux for 3 hours, then ethyl iodide (1.6mol) is added and the mixture stirred under reflux for another 3 hours. To the mixture is added HCl (300ml 31%) and the solution extracted with ethyl acetate (250ml) eight times. The organic layer is washed with saturated brine and dried with Na2SO4. The solvent is distilled away and the product purified by recrystallization to give the desired product in 95.9% yield (0.804mol).
Step Five: Synthesis of E
A solution of sodium methoxide (0.9mol) in tetrahydrofuran (500ml) is cooled to -30*C, and D (0.804mol) in tetrahydrofuran (500ml) is added dropwise with stirring. After the addition, the mixture is stirred at 30*C for 1hr. To the mixture is added acetic anhydride (1.05mol) and the mixture is stirred at room temperature for 3hr. To the reaction mixture is added saturated ammonium chloride (1000ml), and the mixture is extracted with ethyl acetate (800ml). The organic layer is dried (Na2SO4) and the solvent distilled away. The product is recrystallized (ethyl acetate) to give the desired product in 80.0% yeild (0.644mol).
Step Six: Synthesis of F
E (0.644mol) is dissolved into 3-pentanol (600ml) at reflux, and AlCl3 (0.7mol) is slowly added with stirring. The mixture is stirred at reflux for 1 hour, and then allowed to cool to room temperature. A saturated solution of NaHCO3 is added (1000ml), and the mixture is extracted with 750ml DCM, washed with saturated brine (750ml) and dried with Na2SO4. The solvent is distilled away and the product recrystallized (DCM) to give the desired product in 81.2% yield (0.522mol)
Step Seven: Synthesis of G
F (0.522mol) is dissolved in DCM (750ml) and triethylamine (0.78mol) is added. The mixture is stirred at room temperature for 5 min. and then cooled to 0*C. To the mixture is added tosyl chloride (0.78mol) and the mixture is stirred at room temperature for 1hr. The reaction mixture is washed with saturated sodium bicarbonate solution (1500ml), saturated brine (750ml) and then dried with sodium sulfate. The solvent is distilled off, and the crude product recrystallized from DCM to give G in 97.3% yeild (0.508mol).
Step Eight: Synthesis H
G (0.508mol) is dissolved in ethanol (1000ml) at reflux, and potassium carbonate (0.25mol) is added with stirring. The mixture is stirred at gentle reflux for 1.5 hours, and then cooled to room temperature. Saturated ammonium chloride solution (1000ml) is added. The mixture is extracted with DCM (750ml), washed with saturated brine (1000ml) and dried (Na2SO4). The solvent is distilled away and the crude product recrystallized from DCM to give H in 98.5% yeild (0.5mol).
Step Nine: Synthesis of I
H (0.5mol), sodium azide (0.6mol) and ammonium chloride (0.6mol) are dissolved into water (70ml) and ethanol (250ml), and refluxed for 8 hours. Aqeous NaHCO3 (105ml of 8% solution) is added and the ethanol distilled in vacuum. The aqeous residue is extracted with ethyl acetate (250ml), the extract washed with water (125ml). The wash is back extracted with 125ml of ethyl acetate and the combined organic extracts washed with brine (125ml), dried over Na2SO4, filtered and concentrated in vacuum to give I as a dark brown oil in 102% yield (0.512mol)
Step Ten: Synthesis of J
Step One:
J (0.512mol) and ammonium chloride (1.2mol) are dissolved into ethanol (1500ml), and zinc (0.7mol) is added and the mixture refluxed for 30 minutes. The solvent is distilled off to give Ib, which is used directly in the next step.
Step Two:
Tosysl Chloride (1.13mol) is added portionwise at room temperature to a stirred mixture of Ib (from the step one), K2CO3 (2.5mol) in acetonitrile (1000ml) and stirred for 6hr. Toluene (2500ml) is added, the solid is filtered off and the solvent evaporated to give J in approx 80-90% yeild. It is used directly in the next step without purification.
Step Eleven: Synthesis of K
J (0.494mol), sodium azide (1.2mol) and ammonium chloride (1.2mol) in dimethylacetamide (400ml) is heated to 80-85*C for 5 hours. NaHCO3 (50mmol) in water (250ml) is added, and the mixture extracted with hexanes (6x250ml). The combined hexane extracts are concentrated to 1200ml, and 250ml DCM is added, followed by 1100ml NaHCO3 8% solution and acetic anhydride (0.6mol). The mixture is stirred at room temperature for an hour, and then the aqeous layer is removed. The organic phases are concentrated in vacuum to 430g total weight), dissolved in ethyl acetate (50ml). The mixture is cooled and K crystallizes out and is collected by filtering. The crystals are washed with cold 15% ethyl acetate in hexane (250ml) and dried in a vacuum at room temperature, to give K in 55% yield (0.272mol). [may be slighly higher, ie 60%]
Step Twelve: Synthesis of L (oseltamivir freebase)
K (0.272mol) is dissolved into ethanol (600ml) along with ammonium chloride (0.64mol). Zinc (0.36mol) is added and the mixture refluxed for 30 minutes. The precipitate is filtered out, giving L in 98% yeild (0.266mol). [90-95% more likely]
Step Thirteen: Synthesis of Oseltamivir Phosphate
L (0.266mol) is dissolved in acetone (1000ml) and treated with phosphoric acid (85%, 0.266mol) in absolute ethanol (300ml). The mixture is cooled, and after 12 hours the precipitate is filtered out to give oseltamivir phosphate in 75% yield (0.2mol, 82g). A second crop of presumably lower purity crystals can be obtained by concentrated the solution and collecting a second crop of product.
Step One: Synthesis of A
Methyl acrylate (2.78mol) is added to furan (3.95mol) and cooled to -10*C. AlCl3 in DCM (1.15mol in 1-5M conc.) is added slowly with stirring, keeping the temperature below -10*C. The mixture is then cooled with a cold water bath and stirred for 2 hours. The mixture is added to 1000ml of saturated sodium bicarbonate solution, and the precipitate is filtered out. The aqeous layer is extracted twice with 250ml DCM, and the organic layers are combined and dried over anhydrous sodium sulfate. The solvent is distilled away and the product purified by silica gel column chromatography to give the desired product in 38.6% yield (1.07mol).
Step Two: Synthesis of B
A (1.07mol) is added to NaOH (1.65mol, 3 to 15M concentration) in water with stirring, maintaining a temperature of 5*C. The mixture is allowed to warm up over a period of 30 minutes with stirring, and stirred at room temperature for 2 hours. HCl (310ml of 31% sol.) is added to adjust the pH to below 1, and then the mixture is extracted 4 times with 200ml of DCM. The combined organic layers are dried with Na2SO4, and solvent evaporated and the product recrystallized from ethyl acetate to give the desired product in 87.5% yield (0.936mol).
Step Three: Synthesis of C
B (0.936mol) is dissolved into 850ml water, and NaHCO3 (0.936mol) is added slowly, with stirring to control the foaming. After foaming ceases, bromine (0.936mol) is added dropwise with stirring, and then the mixture is stirred at room temperature for 2 hours. The mixture is extracted with 400ml ethyl acetate, washed with 100ml thiosulfate solution (to remove unreacted bromine) and dried with Na2SO4. The solvent is distilled away and the product purfied by recrystallizing from ethyl acetate, giving the desired product in 89.6% yeild (0.84mol)
Step Four: Synthesis of D
Potassium hydroxide (2.5mol) is dissolved into dimethylacetamide (1000ml) and methanol (150ml), and C (0.84mol) is added slowly as the mixture is heated to a gentle reflux. It is stirred under reflux for 3 hours, then ethyl iodide (1.6mol) is added and the mixture stirred under reflux for another 3 hours. To the mixture is added HCl (300ml 31%) and the solution extracted with ethyl acetate (250ml) eight times. The organic layer is washed with saturated brine and dried with Na2SO4. The solvent is distilled away and the product purified by recrystallization to give the desired product in 95.9% yield (0.804mol).
Step Five: Synthesis of E
A solution of sodium methoxide (0.9mol) in tetrahydrofuran (500ml) is cooled to -30*C, and D (0.804mol) in tetrahydrofuran (500ml) is added dropwise with stirring. After the addition, the mixture is stirred at 30*C for 1hr. To the mixture is added acetic anhydride (1.05mol) and the mixture is stirred at room temperature for 3hr. To the reaction mixture is added saturated ammonium chloride (1000ml), and the mixture is extracted with ethyl acetate (800ml). The organic layer is dried (Na2SO4) and the solvent distilled away. The product is recrystallized (ethyl acetate) to give the desired product in 80.0% yeild (0.644mol).
Step Six: Synthesis of F
E (0.644mol) is dissolved into 3-pentanol (600ml) at reflux, and AlCl3 (0.7mol) is slowly added with stirring. The mixture is stirred at reflux for 1 hour, and then allowed to cool to room temperature. A saturated solution of NaHCO3 is added (1000ml), and the mixture is extracted with 750ml DCM, washed with saturated brine (750ml) and dried with Na2SO4. The solvent is distilled away and the product recrystallized (DCM) to give the desired product in 81.2% yield (0.522mol)
Step Seven: Synthesis of G
F (0.522mol) is dissolved in DCM (750ml) and triethylamine (0.78mol) is added. The mixture is stirred at room temperature for 5 min. and then cooled to 0*C. To the mixture is added tosyl chloride (0.78mol) and the mixture is stirred at room temperature for 1hr. The reaction mixture is washed with saturated sodium bicarbonate solution (1500ml), saturated brine (750ml) and then dried with sodium sulfate. The solvent is distilled off, and the crude product recrystallized from DCM to give G in 97.3% yeild (0.508mol).
Step Eight: Synthesis H
G (0.508mol) is dissolved in ethanol (1000ml) at reflux, and potassium carbonate (0.25mol) is added with stirring. The mixture is stirred at gentle reflux for 1.5 hours, and then cooled to room temperature. Saturated ammonium chloride solution (1000ml) is added. The mixture is extracted with DCM (750ml), washed with saturated brine (1000ml) and dried (Na2SO4). The solvent is distilled away and the crude product recrystallized from DCM to give H in 98.5% yeild (0.5mol).
Step Nine: Synthesis of I
H (0.5mol), sodium azide (0.6mol) and ammonium chloride (0.6mol) are dissolved into water (70ml) and ethanol (250ml), and refluxed for 8 hours. Aqeous NaHCO3 (105ml of 8% solution) is added and the ethanol distilled in vacuum. The aqeous residue is extracted with ethyl acetate (250ml), the extract washed with water (125ml). The wash is back extracted with 125ml of ethyl acetate and the combined organic extracts washed with brine (125ml), dried over Na2SO4, filtered and concentrated in vacuum to give I as a dark brown oil in 102% yield (0.512mol)
Step Ten: Synthesis of J
Step One:
J (0.512mol) and ammonium chloride (1.2mol) are dissolved into ethanol (1500ml), and zinc (0.7mol) is added and the mixture refluxed for 30 minutes. The solvent is distilled off to give Ib, which is used directly in the next step.
Step Two:
Tosysl Chloride (1.13mol) is added portionwise at room temperature to a stirred mixture of Ib (from the step one), K2CO3 (2.5mol) in acetonitrile (1000ml) and stirred for 6hr. Toluene (2500ml) is added, the solid is filtered off and the solvent evaporated to give J in approx 80-90% yeild. It is used directly in the next step without purification.
Step Eleven: Synthesis of K
J (0.494mol), sodium azide (1.2mol) and ammonium chloride (1.2mol) in dimethylacetamide (400ml) is heated to 80-85*C for 5 hours. NaHCO3 (50mmol) in water (250ml) is added, and the mixture extracted with hexanes (6x250ml). The combined hexane extracts are concentrated to 1200ml, and 250ml DCM is added, followed by 1100ml NaHCO3 8% solution and acetic anhydride (0.6mol). The mixture is stirred at room temperature for an hour, and then the aqeous layer is removed. The organic phases are concentrated in vacuum to 430g total weight), dissolved in ethyl acetate (50ml). The mixture is cooled and K crystallizes out and is collected by filtering. The crystals are washed with cold 15% ethyl acetate in hexane (250ml) and dried in a vacuum at room temperature, to give K in 55% yield (0.272mol). [may be slighly higher, ie 60%]
Step Twelve: Synthesis of L (oseltamivir freebase)
K (0.272mol) is dissolved into ethanol (600ml) along with ammonium chloride (0.64mol). Zinc (0.36mol) is added and the mixture refluxed for 30 minutes. The precipitate is filtered out, giving L in 98% yeild (0.266mol). [90-95% more likely]
Step Thirteen: Synthesis of Oseltamivir Phosphate
L (0.266mol) is dissolved in acetone (1000ml) and treated with phosphoric acid (85%, 0.266mol) in absolute ethanol (300ml). The mixture is cooled, and after 12 hours the precipitate is filtered out to give oseltamivir phosphate in 75% yield (0.2mol, 82g). A second crop of presumably lower purity crystals can be obtained by concentrated the solution and collecting a second crop of product.
[comment on this post] Append "/@say/your message here" to the URL in the location bar and hit enter.


 Step One: Synthesis of A
Methyl acrylate (2.78mol) is added to furan (3.95mol) and cooled to -10*C. AlCl3 in DCM (1.15mol in 1-5M conc.) is added slowly with stirring, keeping the temperature below -10*C. The mixture is then cooled with a cold water bath and stirred for 2 hours. The mixture is added to 1000ml of saturated sodium bicarbonate solution, and the precipitate is filtered out. The aqeous layer is extracted twice with 250ml DCM, and the organic layers are combined and dried over anhydrous sodium sulfate. The solvent is distilled away and the product purified by silica gel column chromatography to give the desired product in 38.6% yield (1.07mol).
Step Two: Synthesis of B
A (1.07mol) is added to NaOH (1.65mol, 3 to 15M concentration) in water with stirring, maintaining a temperature of 5*C. The mixture is allowed to warm up over a period of 30 minutes with stirring, and stirred at room temperature for 2 hours. HCl (310ml of 31% sol.) is added to adjust the pH to below 1, and then the mixture is extracted 4 times with 200ml of DCM. The combined organic layers are dried with Na2SO4, and solvent evaporated and the product recrystallized from ethyl acetate to give the desired product in 87.5% yield (0.936mol).
Step Three: Synthesis of C
B (0.936mol) is dissolved into 850ml water, and NaHCO3 (0.936mol) is added slowly, with stirring to control the foaming. After foaming ceases, bromine (0.936mol) is added dropwise with stirring, and then the mixture is stirred at room temperature for 2 hours. The mixture is extracted with 400ml ethyl acetate, washed with 100ml thiosulfate solution (to remove unreacted bromine) and dried with Na2SO4. The solvent is distilled away and the product purfied by recrystallizing from ethyl acetate, giving the desired product in 89.6% yeild (0.84mol)
Step Four: Synthesis of D
Potassium hydroxide (2.5mol) is dissolved into dimethylacetamide (1000ml) and methanol (150ml), and C (0.84mol) is added slowly as the mixture is heated to a gentle reflux. It is stirred under reflux for 3 hours, then ethyl iodide (1.6mol) is added and the mixture stirred under reflux for another 3 hours. To the mixture is added HCl (300ml 31%) and the solution extracted with ethyl acetate (250ml) eight times. The organic layer is washed with saturated brine and dried with Na2SO4. The solvent is distilled away and the product purified by recrystallization to give the desired product in 95.9% yield (0.804mol).
Step Five: Synthesis of E
A solution of sodium methoxide (0.9mol) in tetrahydrofuran (500ml) is cooled to -30*C, and D (0.804mol) in tetrahydrofuran (500ml) is added dropwise with stirring. After the addition, the mixture is stirred at 30*C for 1hr. To the mixture is added acetic anhydride (1.05mol) and the mixture is stirred at room temperature for 3hr. To the reaction mixture is added saturated ammonium chloride (1000ml), and the mixture is extracted with ethyl acetate (800ml). The organic layer is dried (Na2SO4) and the solvent distilled away. The product is recrystallized (ethyl acetate) to give the desired product in 80.0% yeild (0.644mol).
Step Six: Synthesis of F
E (0.644mol) is dissolved into 3-pentanol (600ml) at reflux, and AlCl3 (0.7mol) is slowly added with stirring. The mixture is stirred at reflux for 1 hour, and then allowed to cool to room temperature. A saturated solution of NaHCO3 is added (1000ml), and the mixture is extracted with 750ml DCM, washed with saturated brine (750ml) and dried with Na2SO4. The solvent is distilled away and the product recrystallized (DCM) to give the desired product in 81.2% yield (0.522mol)
Step Seven: Synthesis of G
F (0.522mol) is dissolved in DCM (750ml) and triethylamine (0.78mol) is added. The mixture is stirred at room temperature for 5 min. and then cooled to 0*C. To the mixture is added tosyl chloride (0.78mol) and the mixture is stirred at room temperature for 1hr. The reaction mixture is washed with saturated sodium bicarbonate solution (1500ml), saturated brine (750ml) and then dried with sodium sulfate. The solvent is distilled off, and the crude product recrystallized from DCM to give G in 97.3% yeild (0.508mol).
Step Eight: Synthesis H
G (0.508mol) is dissolved in ethanol (1000ml) at reflux, and potassium carbonate (0.25mol) is added with stirring. The mixture is stirred at gentle reflux for 1.5 hours, and then cooled to room temperature. Saturated ammonium chloride solution (1000ml) is added. The mixture is extracted with DCM (750ml), washed with saturated brine (1000ml) and dried (Na2SO4). The solvent is distilled away and the crude product recrystallized from DCM to give H in 98.5% yeild (0.5mol).
Step Nine: Synthesis of I
H (0.5mol), sodium azide (0.6mol) and ammonium chloride (0.6mol) are dissolved into water (70ml) and ethanol (250ml), and refluxed for 8 hours. Aqeous NaHCO3 (105ml of 8% solution) is added and the ethanol distilled in vacuum. The aqeous residue is extracted with ethyl acetate (250ml), the extract washed with water (125ml). The wash is back extracted with 125ml of ethyl acetate and the combined organic extracts washed with brine (125ml), dried over Na2SO4, filtered and concentrated in vacuum to give I as a dark brown oil in 102% yield (0.512mol)
Step Ten: Synthesis of J
Step One:
J (0.512mol) and ammonium chloride (1.2mol) are dissolved into ethanol (1500ml), and zinc (0.7mol) is added and the mixture refluxed for 30 minutes. The solvent is distilled off to give Ib, which is used directly in the next step.
Step Two:
Tosysl Chloride (1.13mol) is added portionwise at room temperature to a stirred mixture of Ib (from the step one), K2CO3 (2.5mol) in acetonitrile (1000ml) and stirred for 6hr. Toluene (2500ml) is added, the solid is filtered off and the solvent evaporated to give J in approx 80-90% yeild. It is used directly in the next step without purification.
Step Eleven: Synthesis of K
J (0.494mol), sodium azide (1.2mol) and ammonium chloride (1.2mol) in dimethylacetamide (400ml) is heated to 80-85*C for 5 hours. NaHCO3 (50mmol) in water (250ml) is added, and the mixture extracted with hexanes (6x250ml). The combined hexane extracts are concentrated to 1200ml, and 250ml DCM is added, followed by 1100ml NaHCO3 8% solution and acetic anhydride (0.6mol). The mixture is stirred at room temperature for an hour, and then the aqeous layer is removed. The organic phases are concentrated in vacuum to 430g total weight), dissolved in ethyl acetate (50ml). The mixture is cooled and K crystallizes out and is collected by filtering. The crystals are washed with cold 15% ethyl acetate in hexane (250ml) and dried in a vacuum at room temperature, to give K in 55% yield (0.272mol). [may be slighly higher, ie 60%]
Step Twelve: Synthesis of L (oseltamivir freebase)
K (0.272mol) is dissolved into ethanol (600ml) along with ammonium chloride (0.64mol). Zinc (0.36mol) is added and the mixture refluxed for 30 minutes. The precipitate is filtered out, giving L in 98% yeild (0.266mol). [90-95% more likely]
Step Thirteen: Synthesis of Oseltamivir Phosphate
L (0.266mol) is dissolved in acetone (1000ml) and treated with phosphoric acid (85%, 0.266mol) in absolute ethanol (300ml). The mixture is cooled, and after 12 hours the precipitate is filtered out to give oseltamivir phosphate in 75% yield (0.2mol, 82g). A second crop of presumably lower purity crystals can be obtained by concentrated the solution and collecting a second crop of product.
Step One: Synthesis of A
Methyl acrylate (2.78mol) is added to furan (3.95mol) and cooled to -10*C. AlCl3 in DCM (1.15mol in 1-5M conc.) is added slowly with stirring, keeping the temperature below -10*C. The mixture is then cooled with a cold water bath and stirred for 2 hours. The mixture is added to 1000ml of saturated sodium bicarbonate solution, and the precipitate is filtered out. The aqeous layer is extracted twice with 250ml DCM, and the organic layers are combined and dried over anhydrous sodium sulfate. The solvent is distilled away and the product purified by silica gel column chromatography to give the desired product in 38.6% yield (1.07mol).
Step Two: Synthesis of B
A (1.07mol) is added to NaOH (1.65mol, 3 to 15M concentration) in water with stirring, maintaining a temperature of 5*C. The mixture is allowed to warm up over a period of 30 minutes with stirring, and stirred at room temperature for 2 hours. HCl (310ml of 31% sol.) is added to adjust the pH to below 1, and then the mixture is extracted 4 times with 200ml of DCM. The combined organic layers are dried with Na2SO4, and solvent evaporated and the product recrystallized from ethyl acetate to give the desired product in 87.5% yield (0.936mol).
Step Three: Synthesis of C
B (0.936mol) is dissolved into 850ml water, and NaHCO3 (0.936mol) is added slowly, with stirring to control the foaming. After foaming ceases, bromine (0.936mol) is added dropwise with stirring, and then the mixture is stirred at room temperature for 2 hours. The mixture is extracted with 400ml ethyl acetate, washed with 100ml thiosulfate solution (to remove unreacted bromine) and dried with Na2SO4. The solvent is distilled away and the product purfied by recrystallizing from ethyl acetate, giving the desired product in 89.6% yeild (0.84mol)
Step Four: Synthesis of D
Potassium hydroxide (2.5mol) is dissolved into dimethylacetamide (1000ml) and methanol (150ml), and C (0.84mol) is added slowly as the mixture is heated to a gentle reflux. It is stirred under reflux for 3 hours, then ethyl iodide (1.6mol) is added and the mixture stirred under reflux for another 3 hours. To the mixture is added HCl (300ml 31%) and the solution extracted with ethyl acetate (250ml) eight times. The organic layer is washed with saturated brine and dried with Na2SO4. The solvent is distilled away and the product purified by recrystallization to give the desired product in 95.9% yield (0.804mol).
Step Five: Synthesis of E
A solution of sodium methoxide (0.9mol) in tetrahydrofuran (500ml) is cooled to -30*C, and D (0.804mol) in tetrahydrofuran (500ml) is added dropwise with stirring. After the addition, the mixture is stirred at 30*C for 1hr. To the mixture is added acetic anhydride (1.05mol) and the mixture is stirred at room temperature for 3hr. To the reaction mixture is added saturated ammonium chloride (1000ml), and the mixture is extracted with ethyl acetate (800ml). The organic layer is dried (Na2SO4) and the solvent distilled away. The product is recrystallized (ethyl acetate) to give the desired product in 80.0% yeild (0.644mol).
Step Six: Synthesis of F
E (0.644mol) is dissolved into 3-pentanol (600ml) at reflux, and AlCl3 (0.7mol) is slowly added with stirring. The mixture is stirred at reflux for 1 hour, and then allowed to cool to room temperature. A saturated solution of NaHCO3 is added (1000ml), and the mixture is extracted with 750ml DCM, washed with saturated brine (750ml) and dried with Na2SO4. The solvent is distilled away and the product recrystallized (DCM) to give the desired product in 81.2% yield (0.522mol)
Step Seven: Synthesis of G
F (0.522mol) is dissolved in DCM (750ml) and triethylamine (0.78mol) is added. The mixture is stirred at room temperature for 5 min. and then cooled to 0*C. To the mixture is added tosyl chloride (0.78mol) and the mixture is stirred at room temperature for 1hr. The reaction mixture is washed with saturated sodium bicarbonate solution (1500ml), saturated brine (750ml) and then dried with sodium sulfate. The solvent is distilled off, and the crude product recrystallized from DCM to give G in 97.3% yeild (0.508mol).
Step Eight: Synthesis H
G (0.508mol) is dissolved in ethanol (1000ml) at reflux, and potassium carbonate (0.25mol) is added with stirring. The mixture is stirred at gentle reflux for 1.5 hours, and then cooled to room temperature. Saturated ammonium chloride solution (1000ml) is added. The mixture is extracted with DCM (750ml), washed with saturated brine (1000ml) and dried (Na2SO4). The solvent is distilled away and the crude product recrystallized from DCM to give H in 98.5% yeild (0.5mol).
Step Nine: Synthesis of I
H (0.5mol), sodium azide (0.6mol) and ammonium chloride (0.6mol) are dissolved into water (70ml) and ethanol (250ml), and refluxed for 8 hours. Aqeous NaHCO3 (105ml of 8% solution) is added and the ethanol distilled in vacuum. The aqeous residue is extracted with ethyl acetate (250ml), the extract washed with water (125ml). The wash is back extracted with 125ml of ethyl acetate and the combined organic extracts washed with brine (125ml), dried over Na2SO4, filtered and concentrated in vacuum to give I as a dark brown oil in 102% yield (0.512mol)
Step Ten: Synthesis of J
Step One:
J (0.512mol) and ammonium chloride (1.2mol) are dissolved into ethanol (1500ml), and zinc (0.7mol) is added and the mixture refluxed for 30 minutes. The solvent is distilled off to give Ib, which is used directly in the next step.
Step Two:
Tosysl Chloride (1.13mol) is added portionwise at room temperature to a stirred mixture of Ib (from the step one), K2CO3 (2.5mol) in acetonitrile (1000ml) and stirred for 6hr. Toluene (2500ml) is added, the solid is filtered off and the solvent evaporated to give J in approx 80-90% yeild. It is used directly in the next step without purification.
Step Eleven: Synthesis of K
J (0.494mol), sodium azide (1.2mol) and ammonium chloride (1.2mol) in dimethylacetamide (400ml) is heated to 80-85*C for 5 hours. NaHCO3 (50mmol) in water (250ml) is added, and the mixture extracted with hexanes (6x250ml). The combined hexane extracts are concentrated to 1200ml, and 250ml DCM is added, followed by 1100ml NaHCO3 8% solution and acetic anhydride (0.6mol). The mixture is stirred at room temperature for an hour, and then the aqeous layer is removed. The organic phases are concentrated in vacuum to 430g total weight), dissolved in ethyl acetate (50ml). The mixture is cooled and K crystallizes out and is collected by filtering. The crystals are washed with cold 15% ethyl acetate in hexane (250ml) and dried in a vacuum at room temperature, to give K in 55% yield (0.272mol). [may be slighly higher, ie 60%]
Step Twelve: Synthesis of L (oseltamivir freebase)
K (0.272mol) is dissolved into ethanol (600ml) along with ammonium chloride (0.64mol). Zinc (0.36mol) is added and the mixture refluxed for 30 minutes. The precipitate is filtered out, giving L in 98% yeild (0.266mol). [90-95% more likely]
Step Thirteen: Synthesis of Oseltamivir Phosphate
L (0.266mol) is dissolved in acetone (1000ml) and treated with phosphoric acid (85%, 0.266mol) in absolute ethanol (300ml). The mixture is cooled, and after 12 hours the precipitate is filtered out to give oseltamivir phosphate in 75% yield (0.2mol, 82g). A second crop of presumably lower purity crystals can be obtained by concentrated the solution and collecting a second crop of product.